The enzyme glutamate dehydrogenase (GDH) functions as a critical part of the metabolic machinery of most cells. This enzyme converts the protein building-block glutamate to α-ketoglutarate, with the help of the small molecule “co-enzyme” NADH. During its catalytic cycle, GDH shifts from an “open” conformation (the catalytic pocket, with binding sites for glutamate and NADH, is open) to a “closed” conformation (the catalytic pocket is closed, and the reaction can take place).
The activity of GDH is regulated not just by the availability of substrate (glutamate) and co-enzyme (NADH), but also by a variety of molecules that bind to an additional “regulatory” site on the enzyme. Some of these molecules act as activators, speeding up GDH activity, while others are inhibitory, slowing down GDH activity. Curiously, NADH can act not just as a co-enzyme, but at high concentrations, it can also bind to the regulatory site and act as an inhibitor of GDH.
In a study published in 2016, we used cryo-electron microscopy to study the effect of NADH binding on the conformation of GDH. Unlike for the completely unbound state (which is open), or one where GDH is bound to glutamate, NADH, and the activator GTP (which is closed), our cryo-EM studies showed that GDH bound by NADH alone can be found in both open and closed states. NADH can be seen occupying the catalytic pocket and the regulatory site in both of these states. NADH can be seen occupying the catalytic pocket and the regulatory site in both of these states, consistent with a key role for NADH binding in the regulation of GDH.
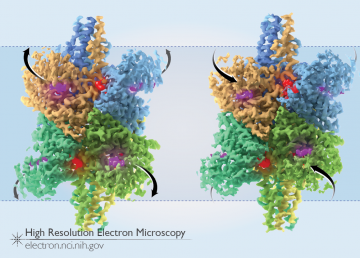
The image above shows the two states of GDH bound to NADH: in the open state (left), the outer domains are lifted out away from the core and the multiple catalytic pockets are open, while in the closed state (right), the outer domains are shifted in towards the core of the molecule and the pockets are closed. Density for NADH in the catalytic pocket is colored in purple, while the density for NADH in the regulatory site is colored in red in each of the 6 GDH subunits. A comparison of the two states of GDH can also be seen in the animation below.
Full-size image: Download (0.7MB) | View animation
Related Reference: Borgnia MJ, Banerjee S, et al. Using cryo-EM to map small ligands on dynamic metabolic enzymes: studies with glutamate dehydrogenase. Mol Pharmacol. 2016 Jun;89(6):645-51. doi: 10.1124/mol.116.103382. [PubMed]