This post is dedicated to the memory of our colleague Soojay Banerjee, the primary author of this study, who passed away October 24, 2017.
While creating new proteins is critical for a cell, getting rid of excess or unneeded proteins is equally critical. This is particularly true for cancer cells, which tend to have higher rates of both protein production and destruction than normal cells. Scientists suspect that if you could impede the protein break-down process, you might be able to kill cancer cells without harming normal cells.
The protein disposal process takes place within the proteasome, a large, barrel-like complex that breaks down proteins. A variety of enzymes can prepare proteins to fit this complex, either by loosening the proteins from other complexes, unfolding them, or directly attaching them to the proteasome.
One of these enzymes is the AAA ATPase p97. p97 processes ATP for energy, and this energy drives successive rotations in the lower and upper sections, followed by a dramatic swing of the outermost subdomain. These movements are necessary for p97’s ability to prepare proteins for the proteasome, and inhibiting p97 may kill cancer cells by preventing protein disposal.
In a recent study, we determined the structure of p97, which revealed the subdomain rearrangements that occur during the ATPase cycle. In addition, we showed that a drug that binds to the interface between the upper and lower sections of p97 acts like a wrench in the works, preventing the enzyme’s ratchet-like rotation.
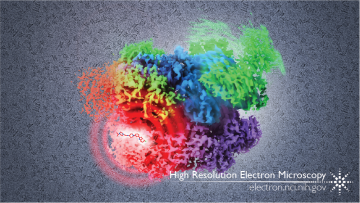
The image above shows a composite of several cryo-EM maps of p97, demonstrating the range of motion of the N-domains (green), as well as the location of the inhibitor (red) between the upper (blue) and lower (purple) ATPase domains. The background shows original cryo-EM images of p97 protein.
Full-size image: Download (1.3MB)
Related Reference: Banerjee S, Bartesaghi A, et al. 2.3 Å resolution cryo-EM structure of human p97 and mechanism of allosteric inhibition. Science. 2016 Feb 19;351(6275):871-5. doi: 10.1126/science.aad7974.