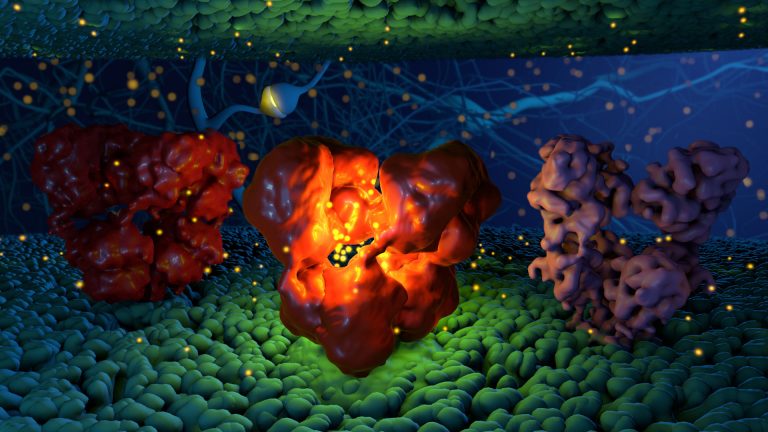
Ionotropic glutamate receptors (iGluRs) belong to a diverse family of channels critical for signaling in the nervous system. Prior to glutamate binding, these receptors sit in a “resting” conformation, ready to receive a signal. Glutamate binding to the receptor causes its channel into the cell to “open”, allowing cations such as sodium and potassium to flow inward across the membrane. After a short (millisecond) moment, the receptor changes to a third conformation, “desensitized”, where it no longer allows cations to pass through the membrane, even though glutamate is still bound.
The iGluRs have a narrow channel region buried in the membrane; above that, on the outside of the cell, there is a layer of 4 ligand binding domains (LBD, where glutamate binds), and on top of that there’s another layer of 4 amino terminal domains (ATD). As the organization of the LBD layer changes, the transmembrane channel below opens and closes.
In a series of 3 studies, we used cryo-electron microscopy to understand the nature of the structural changes that occur during the transition from resting to open to desensitized states in two types of iGluRs, the kainate receptor subtype GluK2 and the AMPA receptor subtype GluA2. In these studies, we showed that glutamate binding triggers the ligand binding domain to close down like a clamshell, opening the channel below. As the receptor transitions to the desensitized state, however, two of the 4 ligand binding domains undergo a large clockwise twist, forming a ring at the top of the channel that closes the channel again.
This image is an artistic representation showing the resting (left), open (middle), and desensitized state (right) of iGluRs on a membrane surface.
Full-size image: Download (1.7MB)
Related References:
Meyerson JR, Chittori S, et al. Structural basis of kainate subtype glutamate receptor desensitization. Nature. 2016 Sep 22;537(7621):567-571. doi: 10.1038/nature19352.
Meyerson JR, Kumar J, et al. Structural mechanism of glutamate receptor activation and desensitization. Nature. 2014 Oct 16;514(7522):328-34. doi: 10.1038/nature13603.
Schauder DM, Kuybeda O, et al. Glutamate receptor desensitization is mediated by changes in quaternary structure of the ligand binding domain. Proc Natl Acad Sci U S A. 2013 Apr 9;110(15):5921-6. doi: 10.1073/pnas.1217549110.